News
In a new paper, Ariel Amir suggests that budding yeast coordinate the replication of their DNA not through size, but by how much they grow over time.(Image courtesy of Stephanie Mitchell/Harvard Staff Photographer)
Ninth graders across the country can recite the basic stages of the cell cycle — growth, DNA replication, division — but the world’s best researchers are still trying to figure out how the thing actually works.
How do cells know when to start and stop growing? How do they know how big to get or when to replicate their DNA? Despite its implications for everything from cancer to brewing, understanding the cell cycle is still an open problem.
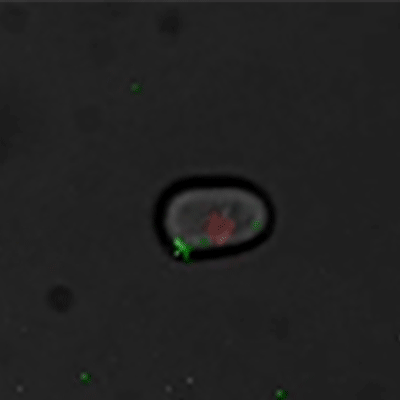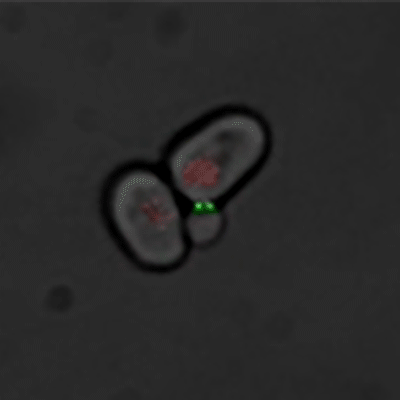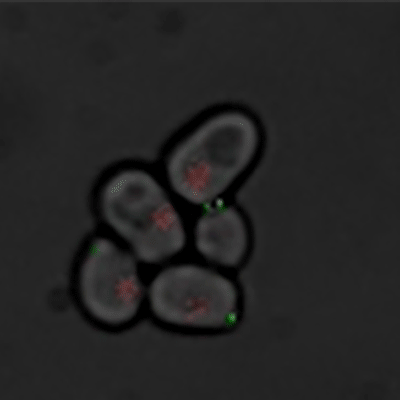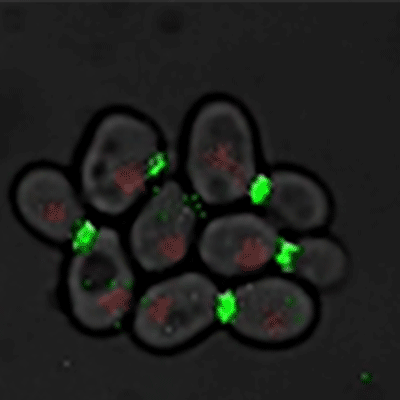
Budding yeast (Image courtesy of Dr. Ilya Soifer)
Ariel Amir, assistant professor in applied mathematics, has spent several years tackling the problem of how cells coordinate cell division. In 2014, he disproved a long held belief that cellular division in bacteria is triggered when cells reach a particular size. Amir suggested that cells coordinate the replication of their DNA not through size, but by how much they grow over time.
In a new paper, Amir observes the same mechanism in budding yeast cells, suggesting that this process may be prevalent across different kingdoms of life. The paper is published in the journal Current Biology.
Amir and his collaborators – Drs. Ilya Soifer and Lydia Robert - found that in order to explain experimental data on cell division in bacteria and yeast, both time and volume have to be considered.
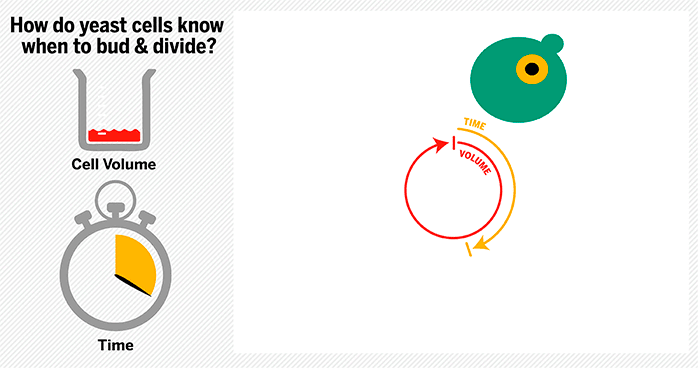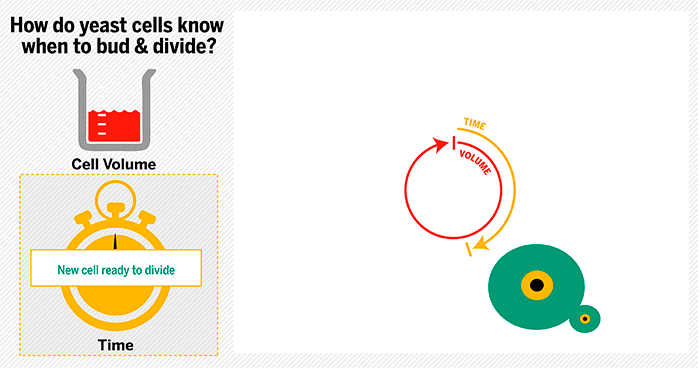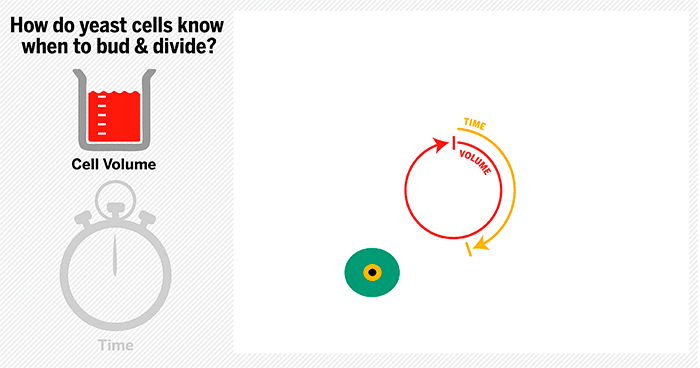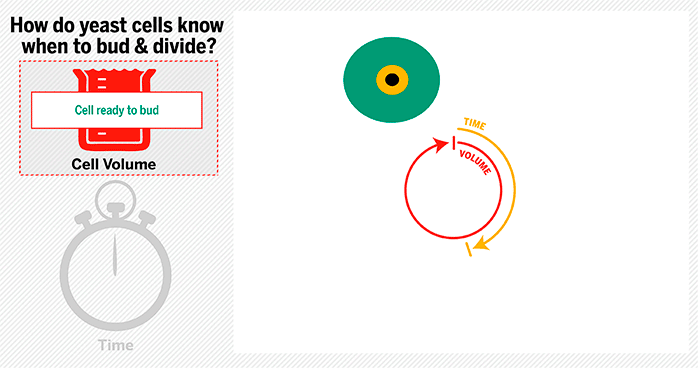
The cycle begins at budding. When a mother cell buds, two gauges begin ticking in the daughter cell, one measuring time, the other volume added. Each gauge has a pre-programmed stopping point. When the timer runs out, the daughter cell knows its time to divide. When the right amount of volume is added, the daughter knows its time to bud. Every cell, regardless of its size at birth, adds the same amount of volume before budding and grows the same amount of time before division.
“This is identical to what we see in bacteria,” Amir said. "This mechanism may have evolved as a robust way to coordinate the various events in the cell cycle - growth, division and DNA replication - using simple biological components."
The next step of the research is to figure out which biological components are involved in regulated the gauges, how prevalent this mechanism is in other organisms and understanding how genetic mutations affect this process.
Topics: Bioengineering, Applied Physics
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Scientist Profiles
Ariel Amir
Associate in Applied Mathematics
Press Contact
Leah Burrows | 617-496-1351 | lburrows@seas.harvard.edu