News
The Mountain Bluebird gets its distinct blue color from nanoscale structures that reflect specific wavelengths of light (Image courtesy of Andrej Chudý/Flickr)
Imagine paint that never fades or a full-color e-reader that is easier to read in the sunlight.
Structural color — color derived from nanoscale structures that reflect specific wavelengths of light — does not fade over time or react to light like pigments. This type of color is abundant in nature, giving many species of birds and insects their vibrant, long-lasting color schemes. Today, most structural color for applications mimics those structures found in nature. But natural structures are complex and mimicking them is complicated and time consuming.
What if there were an easier way to design structural color for human applications?
Now, researchers at the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) have developed a model that can predict what color a certain structure will produce given a set of parameters. Using their model, the researchers recreated the specific sky-blue color of the mountain bluebird using prescribed materials and a simple microstructure.
The research was published recently in the Proceedings of the National Academy of Sciences.
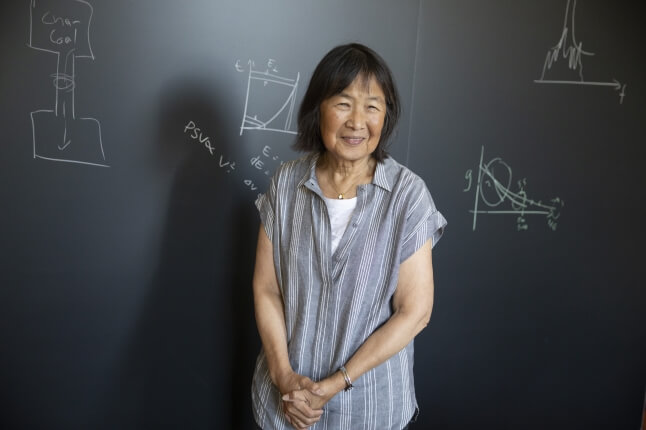
“The structure that makes a bluebird’s wing blue is complex and evolved to meet many different constraints,” said Vinothan Manoharan, the Wagner Family Professor of Chemical Engineering and Professor of Physics at SEAS and senior author of the study. “While biomimicry can teach us ways to make new colors, it’s not necessarily the path we want to follow for particular applications. We showed that you can make the same color by using a simple, easy-to-make structure.”
The research was conducted in collaboration with BASF through Harvard University’s participation in the BASF "Northeast Research Alliance" or NORA, a strategic research alliance among BASF, Harvard, MIT, and the University of Massachusetts at Amherst. The goal of NORA is to facilitate collaboration on research projects to generate a better fundamental understanding in fields of mutual interest to academic scientists and BASF.
“The research on structural color conducted within this framework is a great example of how close collaboration can lead to the exploration of new areas and the advancement of fundamental understanding,” said Rupert Konradi, Head of NORA at BASF.
“We are excited that the collaboration between Harvard and BASF has been successful,” said Rupa Darji, a Technical Manager from the Advanced Materials & Systems Research division at BASF Corporation and principal investigator of the collaboration. “This collaboration utilized the strengths from both academic and industrial scientists. Harvard excelled at their ability to fundamentally understand the physics, and BASF applied their expertise in chemistry and engineering."
We’ve learned a lot from nature but once you learn the physics you can go beyond nature. That’s what human engineers do.

To predict and design structural color, the researchers first had to understand the physics behind the phenomenon.
Victoria Hwang, a former graduate student in the Manoharan lab, developed a model to simulate how light travels through and interacts with a simple nanostructure consisting of spheres embedded in a matrix.
Hwang measures the color of an Abyssinian roller bird specimen from the Museum of Comparative Zoology in the Center for Nanoscale Systems at Harvard. (Photo by Kate Eldrige, Museum of Comparative Zoology, Harvard University)
Hwang and the team looked at two aspects of the physics in particular — constructive interference and multiple scattering.
Constructive interference describes how some wavelengths pass through materials while others are reflected back, giving rise to color. Multiple scattering describes how light bounces around inside a material, which affects the saturation or how vivid the color appears.
“Previous models could tell you that a color is blue-ish, but they couldn’t say what kind of blue,” said Hwang, who is the first author of the paper. “With our model, we can tell you the exact tone of blue as well as its saturation.”
The researchers compared the model to the deep blue they made in experiments and the two matched.
The color can be changed by changing the parameters of the structure, including nanoparticle size, refractive indices of the materials, how tightly the particles are packed and the thickness of the sample. The researchers created angle-independent structural color, meaning matte rather than iridescent colors. To achieve that matte finish, the nanoparticles are disordered.
“Angle-independent structural colors are really useful for industry because they look a lot like dyes and pigments, but they don't need to absorb any light,” said Hwang. “Using angle-independent colors, you could imagine making an e-reader with a full color display so that when you take it outside in the sun it’s actually easier to read because it’s reflecting the light.”
“We want to use the framework we've developed to make useful materials,” said Manoharan. “That’s why we’re excited to be collaborating with BASF. We think our method could be applied to many different materials.”
For example, said Manoharan, if you want to make a colored plastic material today using regular dyes and pigments, you need different molecules for each color. But with structural color, you can use the same component materials for every color and just change the size or density of the particles. The researchers used polystyrene nanoparticles in their experiments, but different industries could use different materials, depending on the needs of the application.
Next, the team hopes to push the boundaries of what’s possible in structural color, aiming to make reds, infrareds and even ultraviolet structures.
“We’ve learned a lot from nature but once you learn the physics you can go beyond nature. That’s what human engineers do,” said Manoharan.
The research was co-authored by Anna Stephenson, Solomon Barkley, Soeren Brandt, Ming Xiao and Joanna Aizenberg, the Amy Smith Berylson Professor of Materials Science and Professor of Chemistry & Chemical Biology.
The research was supported by BASF Corporation and the BASF Northeast Research Alliance; by the Harvard MRSEC under National Science Foundation (NSF) grant no. DMR-2011754; and by the NSF Graduate Research Fellowship under grant no. DGE-1144152.
Topics: Applied Physics, Industry, Materials
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Scientist Profiles
Vinothan N. Manoharan
Wagner Family Professor of Chemical Engineering and Professor of Physics
Press Contact
Leah Burrows | 617-496-1351 | lburrows@seas.harvard.edu