News
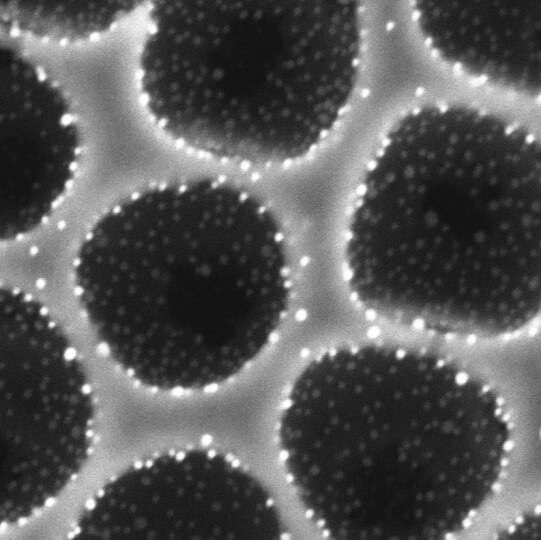
The bioinspired architecture allows precious metal catalysts (white) to be strategically placed on the porous scaffold (gray) so that the catalytic reaction is much more efficient and cost-effective. (Image courtesy of Wyss Institute at Harvard University)
In the late 1700s, a Scottish chemist named Elizabeth Fulhame discovered that certain chemical reactions occurred only in the presence of water and that, at the end of those reactions, the amount of water was not depleted. Fulhame was the first scientist to demonstrate the power of a catalyst — a material that can speed up a chemical reaction without being consumed by it.
Two hundred years later, catalysts one of the engines of modern life. The chemical industry relies on catalysts for 90 percent of its processes — everything from refining oil, turning petroleum into plastic, and producing fertilizers food and medicine, to scrubbing the air of noxious pollutants emitted from cars and factories.
Designing catalytic systems for such a broad range of applications is a big challenge. Catalysts need to be integrated into systems spanning a wide range of sizes, shapes, and material compositions, and control a variety of chemical reactions under vastly different conditions. In addition, most specialized catalysts rely on rare and expensive metals such as platinum, palladium, and rhodium supported on high-surface-area metal or metal oxide matrices.
Now, a team of researchers from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) and the Harvard Wyss Institute for Biologically-Inspired Engineering has developed and tested a new approach to optimize the design of tunable catalytic systems.
One of the biggest challenges in developing effective catalysts is designing the nanostructured porous solids on and in which reactions take place. For a long time, Aizenberg’s research has focused on studying complex natural micro and nanostructured materials — such as those in iridescent opals or in butterfly wings — and unraveling the ways biology controls the chemistry and morphology of its nanoscale building blocks. Inspired by natural processes, the team of researchers at SEAS and Wyss developed a methodology to create perfect, highly ordered, opal-like micromaterials for a wide range of catalytic and photocatalytic reactions.
To create these structures, the researchers introduced a co-assembly method in which tiny, spherical particles and matrix precursors are deposited simultaneously from a single mixture to produce defect-free films over centimeter scales. The researchers demonstrated this process with widely-used catalytic materials, including titania, alumina and zirconia, incorporating various mono and multi-metallic nanoparticles.
“Expanding this methodology to non-biological crystalline materials will result in microscale architectures with enhanced photonic, electronic, and catalytic properties,” said Tanya Shirman, a postdoctoral fellow at SEAS and Technology Development Fellow at Wyss Institute and co-author of the research.
In the design of the catalytic particles themselves, the researchers also turned to nature, using bio-catalysts, such as enzymes, for inspiration. In biological systems, the nanoscale catalytic materials attach to larger entities such as proteins and cells, which self-organize to form larger networks of precisely designed catalytic sites.
By optimizing the design and minimizing the amount of precious metals used in catalytic systems, we can create more sustainable catalysts in general and use catalytic materials in ways that are not currently affordable.
“Nature has had billions of years of R&D to perfect the design of catalytic systems,” said Tanya Shirman. “As a result, they are incredibly efficient and enable the coordination and fine-tuning of sophisticated reactions through optimal positioning of the catalytic complexes.”
The researchers mimicked the hierarchical architecture of natural catalysts by developing a highly modular platform that builds complex catalysts from organic colloids and inorganic catalytic nanoparticles. The team can control everything from the composition, size, and placement of the catalytic nanoparticles to the colloid size, shape, and connectivity, and the overall shape and patterns of the network. The resulting catalytic systems use significantly lower amounts of precious metals than existing catalysts.
“Precious metal is a very limited resource,” said Elijah Shirman, a postdoctoral fellow at SEAS and Wyss Institute and co-author of the research. “By optimizing the design and minimizing the amount of precious metals used in catalytic systems, we can create more sustainable catalysts in general and use catalytic materials in ways that are not currently affordable.”
The method is relatively simple: First, the catalytic nanoparticles attach to the colloids through various kinds of chemical and physical bonding. Coated with nanoparticles, the colloids are next placed into a matrix precursor solution and allowed to self-assemble into the desired pattern, which can be controlled by confining the assembly within a certain shape. Lastly, the colloids are removed so that a structured network that is decorated with nanoparticles partially embedded inside the matrix is formed. This hierarchical porous architecture with firmly attached catalytic sites maximizes the surface area for the catalytic reaction and enhances the robustness of the catalyst.
“Our synthetic platform allows one to take the components of the assembly and form a fully interconnected, highly ordered porous microarchitecture, in which catalytic nanoparticles are uniquely incorporated,” said Tanya Shirman. “This provides exceptional mechanical, thermal, and chemical stability as well as high surface area and full accessibility to diffusing reactants.”
“The technology developed in my lab is particularly promising for bridging the gap between state-of-the-art R&D and real-world applications,” said Joanna Aizenberg. “Due to its modular design and tunability, this framework can be used in various fields from the synthesis of important chemical products, to pollution abatement. Our results clearly show that we are now able to create better catalysts, use less precious metal and improve known catalytic processes.”
This technology is now being validated and developed for commercialization by the Wyss Institute.
Joanna Aizenberg, Amy Smith Berylson Professor of Materials Science and Professor of Chemistry & Chemical Biology (Image courtesy of Stephanie Mitchell/Harvard University News Office)
Aizenberg’s team is currently focusing on developing next-generation catalysts for a number of applications – from clean air technologies and catalytic converters to advanced electrodes for catalytic fuel cells – hoping to test their designs soon in real world systems.
The team recently received second place in Harvard’s President’s Innovation Challenge, which identifies and promotes promising technology enterprises that have the potential for significant societal and environmental impacts.
The research was co-authored by Cynthia Friend, Theodore William Richards Professor of Chemistry and Professor of Materials Science at SEAS; Anna V. Shneidman; Alison Grinthal; Katherine R. Phillips; Hayley Whelan; Eli Bulger; Marcus Abramovitch; Jatin Patil; Rochelle Nevarez; Theresa M. Kay Judith Lattimer; Mathilde Luneau; Christian Reece; Michael Aizenberg; and Robert Madix.
This work was supported by the National Science Foundation Designing Materials to Revolutionize and Engineer our Future program under award #DMR 1533985 and in part by the Integrated Mesoscale Architectures for Sustainable Catalysis, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under award #DE-SC0012573.
Topics: Materials
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Scientist Profiles
Joanna Aizenberg
Amy Smith Berylson Professor of Materials Science and Professor of Chemistry & Chemical Biology
Press Contact
Leah Burrows | 617-496-1351 | lburrows@seas.harvard.edu