News
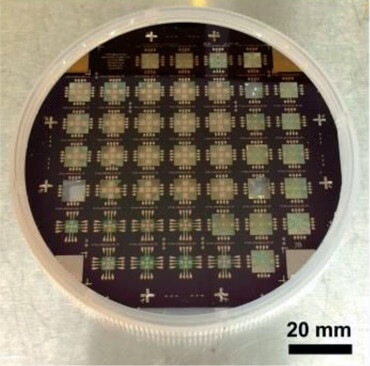
Image of the sensor array (Image courtesy of Harvard SEAS)
Download ImageResearchers at the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) have developed a sensor that can measure biological samples as small as a single cell, offering a previously unseen window into the energetics of cellular processes.
The research was published in Advanced Science.
“Living organisms use energy to grow, reproduce, and interact with their environment,” said Joost Vlassak, the Abbott and James Lawrence Professor of Materials Engineering at SEAS, and senior author of the paper. “The energy expenditure associated with these processes is a critically important parameter in biology and provides a probe into some of the most fundamental processes that keep an organism alive. Up until now, it has been very difficult to measure energy expenditure of small organisms directly as the temperature differences involved are extremely small. Instead, researchers use indirect methods such as oxygen or glucose consumption, or metabolite production.”
Calorimetry, the science of measuring the heat exchanged between a system and its environment as a result of chemical reactions, has long been used as a research tool in fields ranging from pharmacology and biology to materials science. Calorimetry techniques typically require relatively large samples, as well as long measurement times. As a result, they lack the sensitivity needed to measure the energetics of small-scale systems, such as a single mouse egg — until now.
Vlassak and his team developed a calorimetry sensor that has a sensitivity in the picowatt range, that can make measurements on biological samples as small as a single cell, and has a response time on the order of seconds.
“These attributes make it possible to directly measure metabolic rates of biological systems at unprecedented scales,” said Juanjuan Zheng, a postdoctoral fellow at SEAS and co-author of the paper.
The sensor consists of a set of low-noise thermopiles on a thin silicon nitride membrane that allow direct differential temperature measurements between a sample and four coplanar references. Using a design with four references makes it possible to eliminate the effects of any in-plane temperature gradients that may arise during the measurement as a result of small changes in the ambient temperature. A hydrogel-lined enclosure is used to prevent evaporation of samples without direct thermal contact with the sample. The resulting sensor has a resolution that is about an order of magnitude higher than the most advanced calorimeter sensors.
“This extraordinary sensitivity makes the sensor ideally suited for the study of processes in biological systems, such as early embryonic development,” said Zheng.
“There is a long history of studying energetics at the organismal level – for example, the efficiency of walking and swimming – which has provided great insight into the behavior and evolution of animals,” said Daniel Needleman, Gordon McKay Professor of Applied Physics and Professor of Molecular and Cellular Biology and co-author of the paper. “However, we know almost nothing about the energetics of the fundamental cellular process that underlie all of life: how much energy does it take to synthesize a protein or assemble an organelle? Do cells use more energy to move and change their shape, or to process information? What aspects of cells are efficient and what aspects are wasteful? The reason that these, and similar questions, have remained unanswered is because of a lack of tools capable of making the relevant measurements. Because of its unprecedented sensitivity, this new sensor will enable researchers to start unraveling the mysteries of cellular energetics. I really believe that this has the potential to help reveal fundamental principles of life.”
The research team has already begun collaborating with other groups to make measurements.
“We hope to make this tool available to as many research groups as possible,” said Vlassak. “We are also exploring ways to make the sensor even more sensitive. With the current sensor, we can measure metabolic rates in very small organisms or in large single cells such as oocytes, but many cells are significantly smaller and have much lower metabolic rates – these still present a challenge.”
Harvard’s Office of Technology Development (OTD) has protected the intellectual property associated with this project and is exploring commercialization opportunities.
This research was co-authored by Jinhye Bae, Haitao Zhang, and Peter J. Foster.
It was supported by the Harvard University MRSEC, which is funded by the National Science Foundation under Grants DMR-1420570 and DMR-2011754. Part of this work was performed at the Center for Nanoscale Systems (CNS), which is supported by the National Science Foundation under Grant ECS 1541959.
Topics: Materials, Technology
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Scientist Profiles
Joost J. Vlassak
Abbott and James Lawrence Professor of Materials Engineering
Daniel Needleman
Gordon McKay Professor of Applied Physics and Professor of Molecular and Cellular Biology
Press Contact
Leah Burrows | 617-496-1351 | lburrows@seas.harvard.edu