News
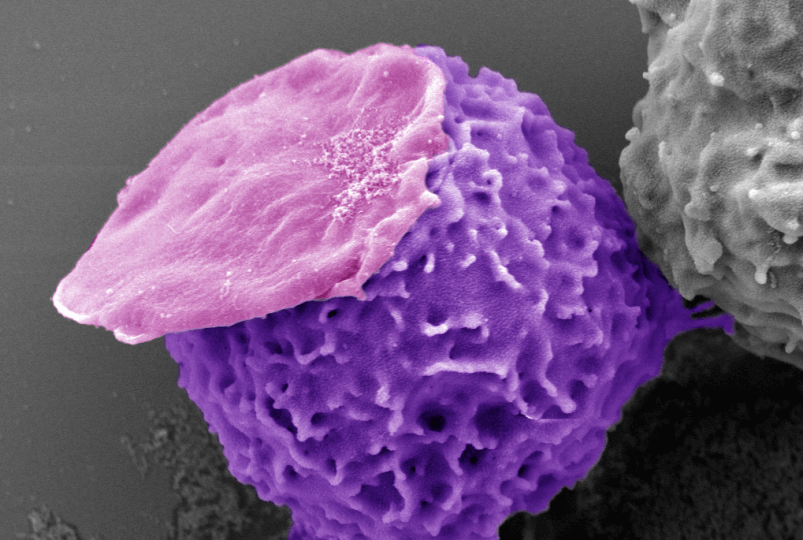
A neutrophil (purple) carries an empty "backpack" (pink) made of polymers. Credit: Wyss Institute at Harvard University
Most of the white blood cells in your body are a type of cell called neutrophils. Despite their high numbers, they are less well understood than other immune cells, in part because they have very short lifespans: an average neutrophil lives for only eight hours. However, recent work has shown that neutrophils are very flexible cells, capable of dialing inflammation up or down, especially in the context of cancer. This makes them attractive targets for immunotherapy, which aims to tweak the immune system to more potently attack disease. But neutrophil-based therapies are still in their infancy.
A team from the Harvard’s John A. Paulson School of Engineering and Applied Sciences (SEAS) and the Wyss Institute at Harvard University and is helping advance neutrophil therapies to the crawling stage. In a recent paper published in Nature Biomedical Engineering, the researchers demonstrated that by attaching disc-shaped microparticles called “backpacks” to neutrophils, they could maintain the cells in their anti-tumor (N1) state. When these treated neutrophils were infused into mice with cancer, they activated other immune cells against the disease, including natural killer (NK) cells and T cells. Treated mice had smaller tumors and lived longer than untreated mice, and the results were even better when the backpack-bearing neutrophils were combined with common “checkpoint inhibitor” cancer drugs.
“Unlike in previous work from our lab where we filled the backpacks with stimulatory molecules that were delivered into immune cells to activate them, we discovered that just the act of attaching “empty” backpacks to neutrophils is enough to activate them – making this technique attractive as a “drug-free” cell therapy for cancer,” said first author Ninad Kumbhojkar, a former graduate student at SEAS and Wyss, who is now a scientist at Tessera Therapeutics.
Teaching an old backpack new tricks
Neutrophils have been used for more than 40 years to help boost the immune systems of cancer patients who are receiving chemotherapy, which depletes their bodies’ ability to fight infections. But deploying neutrophils to directly target cancer itself is uncharted territory that Kumbhojkar and his co-authors decided to explore, using the backpack technology pioneered in the lab of Samir Mitragotri, Hiller Professor of Bioengineering and Hansjörg Wyss Professor of Biologically Inspired Engineering at SEAS.
Backpack microparticles have been previously used to influence the behavior of immune cells called macrophages, successfully activating them to treat cancer, multiple sclerosis, and traumatic brain injury in animal models. Recent research has revealed that neutrophils are similar to macrophages in that they can be in either a pro-inflammatory or anti-inflammatory state. Since they could successfully control macrophages’ state with their backpacks, the researchers had a hunch that the approach should also work on neutrophils.
When neutrophils physically encounter a foreign object that is too large for them to engulf, such as a splinter or a surgical implant, they undergo a series of biological changes that alert the body’s immune system to the threat. The researchers had to design a backpack that was large enough to trigger this response, but small enough to move with the neutrophils and not impede their normal functions. They crafted a new type of backpack they called Cyto-Adhesive Micro-Patches, or CAMPs: disc-shaped microparticles composed of a blend of two polymers. Unlike previous backpacks, CAMPs do not contain any biologically active chemicals designed to be delivered into their host cells. To help the CAMPs attach to the neutrophils, they added a fragment of an antibody that would bind to a specific protein found on the cells’ surfaces.
The researchers mixed CAMPs with fresh mouse neutrophils, and saw that they readily stuck to the cells without harming them. CAMP-bearing neutrophils rapidly released several molecules indicative of activation against immune threats, both in the lab and when injected into mice. They also found that CAMPs caused over 4,000 genes in the neutrophils to be expressed differently than untreated neutrophils. Specifically, neutrophils with CAMPs expressed more genes associated with the anti-tumor state, as well as genes coding for pro-inflammatory cytokines. When co-cultured with other immune cells including CD8+, T cells, NK cells, dendritic cells, and macrophages, CAMP-bearing neutrophils activated them as well.
When they performed similar experiments with human neutrophils in vitro, they observed similar effects, suggesting the results could translate to humans.
The tumor test
The CAMPs now faced the ultimate test: keeping neutrophils activated in the presence of immune-suppressive cancer tumors in vivo. When CAMP-bearing neutrophils were injected into mice with melanoma, the levels of multiple inflammatory cytokines in the animals’ blood increased, and imaging tests showed that the neutrophils migrated to the site of the tumor within four hours (data that were replicated in a breast cancer model). Neutrophils were also observed in high numbers in the animals’ spleens and tumor-draining lymph nodes, and CD8+, T cells, and NK cells in the blood, spleen, and tumors were activated. None of these results were observed in mice treated with non-CAMP neutrophils.
The mice with melanoma that received CAMP-bearing neutrophils displayed much slower tumor growth rates, and mice with breast cancer that received the treatment had a longer survival time, with complete regression of cancer achieved in 15% of the animals.
When the researchers combined their neutrophil treatment with a standard checkpoint inhibitor drug for cancer (aCTLA-4), mice with melanoma displayed significantly slowed tumor growth and improved survival rates. Sixty-seven percent of the animals survived beyond 40 days, and 33% achieved complete remission within 60 days. When these mice were challenged with another melanoma tumor, none of them developed cancer a second time, indicating that their immune systems had generated a robust anti-tumor memory response.
“Part of the beauty of this neutrophil-based cancer treatment is that it does not require any genetic engineering or modification of the therapeutic cells before they are infused, which can greatly cut down on the processing time for getting lifesaving treatments into patients’ bodies,” said senior author Mitragotri, who is also a Wyss Core Faculty member. “It could even be administered at a patient’s bedside within hours, compared to the 14-16 days required for current cell therapy manufacturing techniques.”
The researchers point out that they only incubated neutrophils with CAMPs for two hours before infusing them into mice, and that further studies are needed to determine how long the treated cells can be maintained before infusion. Members of the Mitragotri lab are continuing to explore neutrophils’ ability to treat other cancers including glioblastoma, as well as using their backpacks to sandwich different immune cell types together to improve their delivery to their target tissues.
This research was supported by Harvard SEAS, the Wyss Institute for Biologically Inspired Engineering, and the National Science Foundation Graduate Research Fellowship under Grant No. 1122374.
Additional authors of the paper include Supriya Prakash, Tatsuya Fukuta, Kwasi Adu-Berchie, Neha Kapate, Rocky An, Solomina Darko, Vineeth Chandran Suja, Kyung Soo Park, Alexander Gottlieb, Michael Griffith Bibbey, Malini Mukherji, Lily Li-Wen Wang, and David Mooney, Robert P. Pinkas Family Professor of Bioengineering at SEAS and Wyss Core Faculty member.
Topics: Bioengineering
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Scientist Profiles
Samir Mitragotri
Hiller Professor of Bioengineering and Hansjorg Wyss Professor of Biologically Inspired Engineering
Press Contact
Leah Burrows | 617-496-1351 | lburrows@seas.harvard.edu