News
Key takeaways
- Hydrocephalus is a life-threatening condition in which excess cerebrospinal fluid collects in the brain.
- The condition is usually treated through surgical implantation of brain shunts, which often fail and need to be repositioned or replaced due to infection.
- SEAS researchers developed a computational model that simulates fluid flow around brain shunts.
- The research could support the design of custom shunts for individual patients.
Millions of people worldwide suffer from hydrocephalus, or a buildup of excess cerebrospinal fluid in the brain, and which recently received greater attention when Billy Joel announced his diagnosis. Treatment usually involves surgical placement of shunts to divert fluid away, but this procedure often leads to complications, infections, and multiple re-treatments.
Bioengineers in the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) have developed a new computational model that combines brain anatomy, fluid flow, and biomolecular transport dynamics to simulate shunt performance with pinpoint accuracy. Their goal: help with the creation of custom shunts that are tailored to specific patients’ anatomy and needs.
The work was supported by federal funding from the National Science Foundation and published in Proceedings of the National Academy of Sciences. It was led by SEAS postdoctoral fellow Haritosh Patel, who works in the labs of Joanna Aizenberg, the Amy Smith Berylson Professor of Materials Science at SEAS and Professor of Chemistry and Chemical Biology; and Venkatesh Murthy, the Raymond Leo Erikson Life Sciences Professor of Molecular and Cellular Biology and Paul J. Finnegan Family Director of the Center for Brain Science.
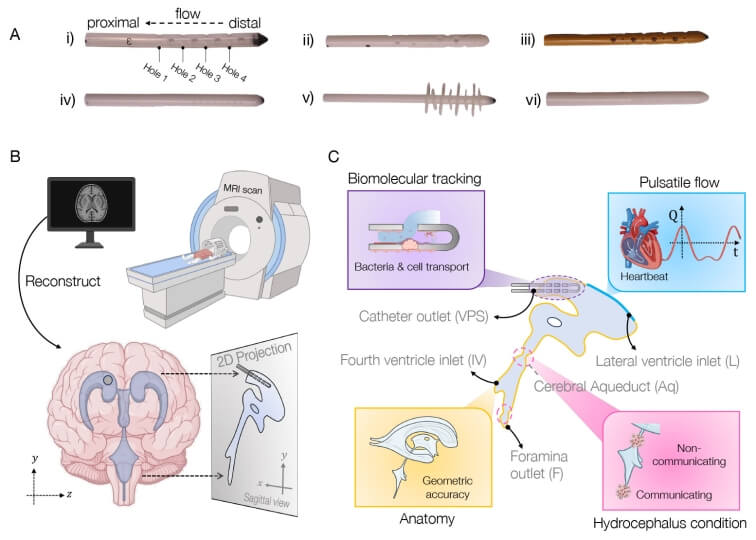
Schematic of approach to simulating brain shunt fluid dynamics.
Repeat surgeries due to infection or obstruction
Tens of thousands of shunt procedures are performed annually in the U.S. — many of which are repeat surgeries due to the inserted devices becoming blocked or obstructed, or the patient suffering an infection.
“Some elderly patients told me they had had over 10 surgeries — one every two to three years,” Patel said. “We really wanted to understand why this was happening, and we realized that many of these obstructions and infections were tied to shunt designs that didn’t fully consider fluid dynamics as a fundamental part of their geometry. We noticed that the tubing geometry used in shunts closely resembles the kind of piping we rely on in household plumbing. While that simplicity has its advantages, we saw an opportunity to explore more creative, biomimetic solutions that better suit the complexity of the brain’s environment.”
Pursuing the problem from both a material and design perspective, the team quickly realized there was no universally accepted fluid flow model for the brain ventricle space to guide them. “Okay, well, we can’t test our devices in a model, so why don’t we first make a better model?” Patel said.
Computational tool simulates fluid flow in brain
The result is their computational tool, called BrainFlow, which combines detailed anatomical and physiological features of the brain to simulate the flow of cerebrospinal fluid flow in the presence of shunt implants.
The model incorporates patient-specific medical imaging data along with pulse-induced flow to mimic a patient’s cerebrospinal fluid dynamics, all to offer insight into optimal shunt design, placement, and even choice of materials.
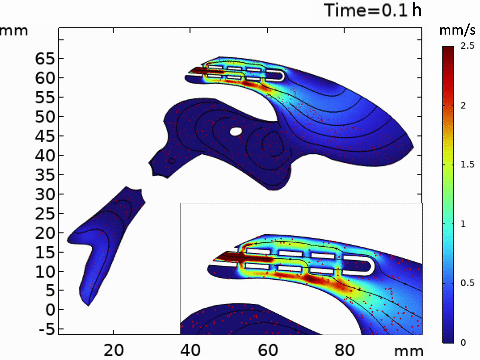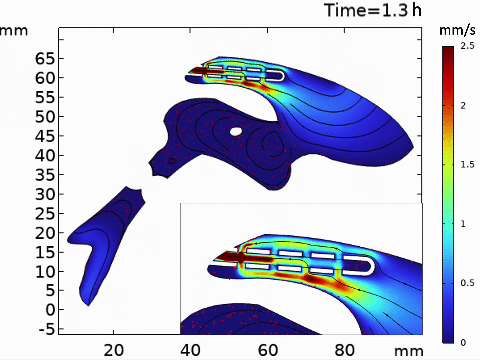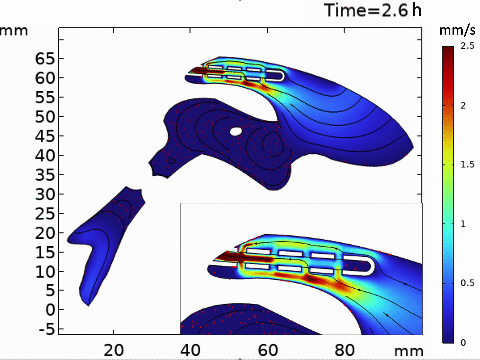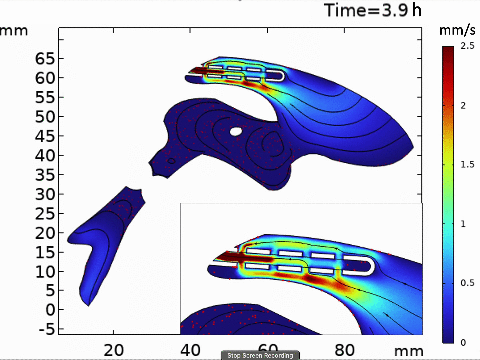
Short-term simulation, using BrainFlow, of cerebrospinal fluid-driven biomolecular transport through a shunt.
“We believe that our model, combined with novel geometries and materials improvements such as anti-biofouling coatings developed in my lab, could lead to smoother integration of optimized, patient-specific medical devices into patients’ brains, with less likelihood of complications, and a better quality of life,” Aizenberg said.
The Harvard team is currently conducting studies that use the model to test different designs of shunts and calculate their efficacy.
The team was supported in part by the Hydrocephalus Association, Hydrocephalus Canada, Hydrocephalus Society, Pediatric Hydrocephalus Foundation, and Society into Research for Hydrocephalus and Spina Bifida. The NSF grant was through the Harvard University Materials Research Science and Engineering Center under award DMR-2011754.
Topics: Bioengineering, Health / Medicine, Materials Science & Mechanical Engineering, Research
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Scientist Profiles
Joanna Aizenberg
Amy Smith Berylson Professor of Materials Science and Professor of Chemistry & Chemical Biology
Press Contact
Anne J. Manning | amanning@seas.harvard.edu