News
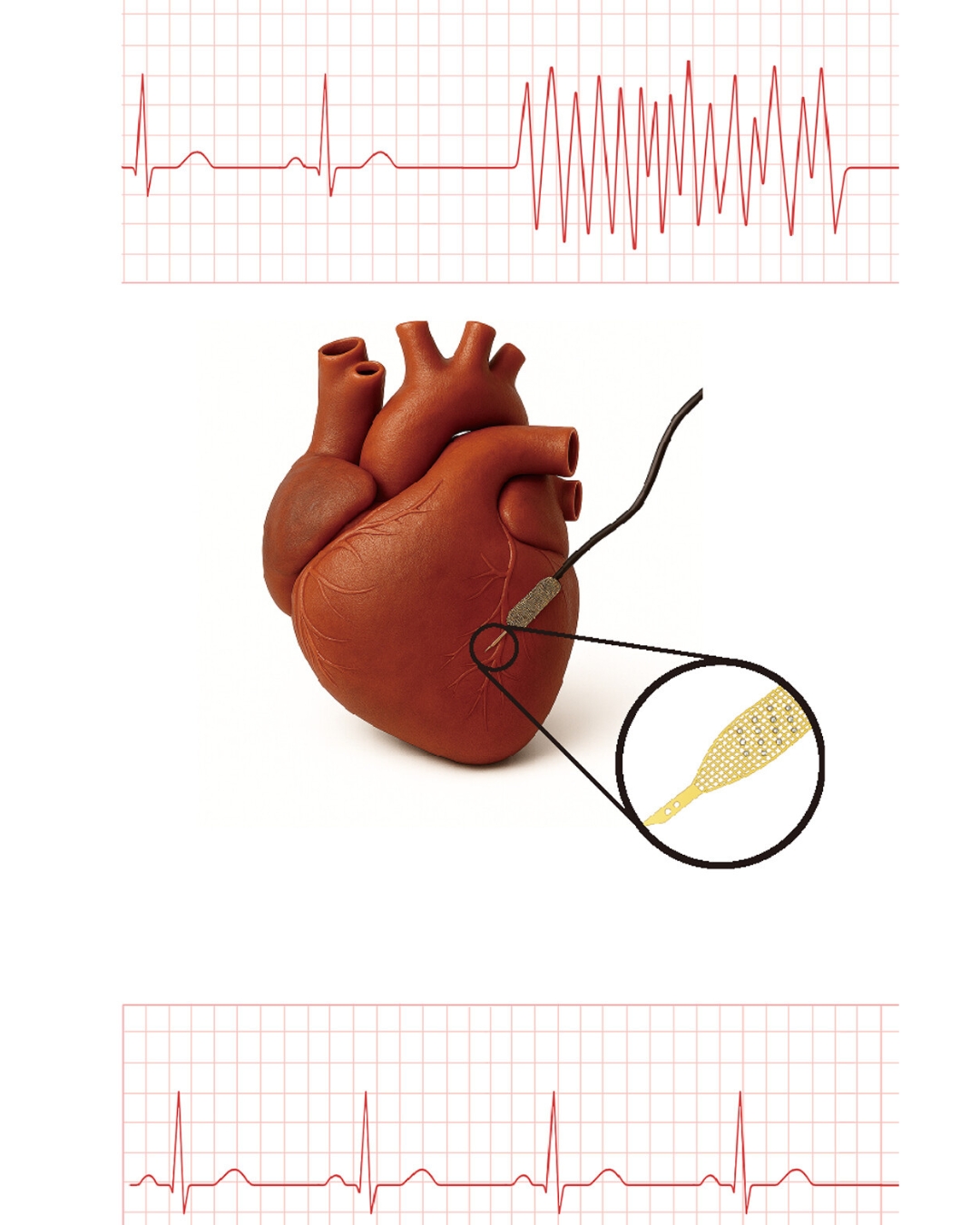
Heart muscle cells grown from patient stem cells — known as human induced pluripotent stem cell–derived cardiomyocytes, or hiPSC‑CMs — are a promising way to repair hearts damaged by heart attacks and heart failure. But transplanted hiPSC‑CMs often have trouble syncing to the rhythm of native heart cells, which can cause dangerous arrhythmias after transplantation.
For years, stem cell biologists and cardiac researchers have been looking for ways to improve how implanted hiPSC‑CMs mature and integrate into the heart. The challenge is that once the hiPSC‑CMs are implanted in vivo, it’s hard to monitor how they integrate.
Now, Harvard University researchers have developed the first platform capable of continuously monitoring how transplanted cells mature, communicate, and synchronize with native tissue inside the body. Using this system, the researchers identified a self-assembling peptide that accelerated the maturation of hiPSC‑CMs and improved the electrical coupling of the transplanted cardiac organoids.
The research was published in Science.
The research was a collaboration between bioengineers at the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), led by Assistant Professor Jia Liu, and stem cell biologists at Harvard’s Department of Stem Cell and Regenerative Biology (SCRB), led by Professor Richard T. Lee.
The platform uses so‑called cyborg organoids developed in Liu’s lab. These organoids are grown with stretchable nanoelectronics embedded in their three-dimensional architecture, allowing for real‑time monitoring and recording of electrophysiological activity. Using this technology, the researchers grew cyborg hiPSC‑CMs with embedded sensors and implanted them in vivo.
Using this system, the researchers were able to isolate the electrical signatures of the transplanted cells from the much stronger signals of a beating heart and pinpoint the exact cells that fell out of sync.
“Until now, no technique could directly reveal what was happening inside at high resolution,” said Liu. “By combining our flexible mesh nanoelectronics with computational analysis, we were able to record, in real time and at cellular resolution, the electrical activity of these transplanted cells within the beating heart.”
With this platform, Lee’s team tested different strategies to improve the maturation of transplanted cells, including a self‑assembling peptide called RADA16, which is already approved as a hemostatic agent.
RADA16 forms small, supportive fibers similar to the heart’s natural environment. Mixed with stem cell-derived cardiomyocytes before transplantation, RADA16 acted as a scaffold, dramatically improving the cells’ ability to integrate into host tissue.
The team used the mesh electronics to monitor the transplanted hiPSC‑CMs for months and found that the addition of RADA16 dramatically changed the behavior of the transplanted cells. Compared to patches without the peptide scaffold, the RADA16-treated hiPSC-CMs showed more mature structural organization, stronger electrical coupling with host tissue, and far fewer signs of arrhythmia-like asynchronous activity over months of monitoring.
Instead of pockets of transplanted cells firing at their own pace, the RADA16-supported patches were largely in sync to the rhythm of the native heart.
“This work demonstrates how flexible bioelectronics and cyborg organoids together can form a powerful framework for assessing the safety and efficacy of regenerative medicine,” said Liu.
“Like all new therapies, safety is a big issue for human heart cell therapy,” said Lee. “We think this approach may help the field identify the safest approach."
This approach could also be used for other types of regenerative therapies where transplanted cells need to wire into existing tissue.
The research was supported in part by the National Institutes of Health.
It was co‑authored by Junya Aoyama, Ren Liu, Xinhe Zhang, Anthony Y. Zhu, Pichayathida Luanpaisanon, Nivedhitha Velayutham, Jessica C. Garbern, Fang Cao, Irving Barrera, Hannah Fandl, Morgan Sokol, Satvik Dasariraju, Eun Seok Gil, Elton Aleksi, Toshi Amanuma, Jeffrey J. Saucerman, and Fei Chen.
Topics: Bioengineering, Electrical Engineering, Health / Medicine, Research
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Scientist Profiles
Jia Liu
Assistant Professor of Bioengineering
Press Contact
Leah Burrows | 617-496-1351 | lburrows@seas.harvard.edu